COVID-19 Vaccine Clinics for First Responders Updated BOH FAQs (UPDATED April 7, 2021)
Update, April 7, 2021: The Department of Public Health’s Office of Emergency Medical Services (Department) has issued an updated Special Protocol for EMT-Basic COVID-19 Vaccine Administration. See attached. The sole update is to allow EMT-Basics, only when they are administering COVID-19 vaccine to an individual in their home, to receive their supervision and oversight from a physician, nurse or paramedic who is immediately available by phone or video. Otherwise, as before, all EMT-Basics vaccinating under the Commissioner’s Order must be working with at least one physician, nurse, or paramedic immediately available at the treatment site as the supervisor of dosing, procedure, and technique.
For any questions with regard to the temporary authorization for EMS personnel to administer COVID-19 vaccine, please contact renee.atherton@mass.gov.
This guidance answers commonly asked questions we have received from Local Boards of Health to support COVID-19 vaccine clinics for First Responders. Topics covered in this guidance include:
Vaccine providers can also refer to www.mass.gov/CovidVaccineProviders for additional information, including detailed Guidance on COVID-19 Vaccine Management and Administration for Healthcare Providers and Organizations and frequently asked questions from vaccine providers.
Timing and Populations to be Vaccinated
When will we be expected to stand up the first responder clinics?
Based upon current expectations of vaccine availability, you and your partners should be prepared to start scheduling of appointments during the week of January 4, 2021 and stand up your initial clinics during the week of January 11, 2021. Vaccination may not begin before January 11, 2021.
Who will be eligible to receive the COVID-19 vaccine at these clinics?
Emergency Medical Services (EMS), police, and fire personnel are eligible for vaccinations in the first responder clinics during Phase 1. This includes all interfacility transport workers, MedFlight staff, college/university campus police, and 911 Dispatch employees. Visit When can I get the COVID-19 vaccine? | Mass.gov often as the priority groups are updated frequently. These clinics will not be open to family or household members who are not currently employed as first responders. Please note that the vaccination needs of State Police will be addressed separately and are not included in these locally organized clinics.
Individuals seeking vaccination are required to provide documentation of eligibility, as described at COVID-19 Vaccine Locations for First Responders | Mass.gov. This page also includes a map of COVID-19 vaccine locations with contact details and sign-up information for First Responders.
May we vaccinate the vaccinators and administrative staff who will support clinic operations?
Yes, clinical staff who will be administering vaccine and support staff who will be patient-facing may be vaccinated. Staff who are assigned to positions in which they will not have direct contact with individuals receiving vaccine are not eligible. Please note that after patient-facing staff receive the initial dose of vaccine, they must continue to use appropriate personal protective equipment (PPE) at all times and practice hand hygiene and other health precautions.
If first responders choose not to get vaccinated at these clinics, will vaccine be available when they are ready?
Individuals in a priority group remain eligible during their phase of COVID-19 vaccination and any time thereafter. First responders are urged to access COVID-19 vaccine as soon as they are eligible. First responders will continue to have access to the vaccine through other sites, such as mass vaccination sites.
Will private ambulance services be vaccinating their own employees?
There may be some private ambulance services that will request vaccine directly for their employees. However, this is not the case for all services and any community that has arranged to vaccinate their private ambulance services should expect to continue with that arrangement.
Are we allowed to vaccinate other COVID-facing healthcare workers at these clinics? (New 1/13/21)
Yes, LBOH clinics are encouraged to vaccinate COVID-facing healthcare workers if they have the capacity. COVID-facing healthcare workers are required to provide documentation of eligibility, as described at COVID-19 vaccine locations for individuals currently eligible to be vaccinated | Mass.gov and should verify they are eligible for vaccine as a COVID-facing health care worker.
To ensure enough vaccine allocation for your site, sites will need to complete a weekly MCVP survey to ensure that DPH understands providers’ vaccine needs, the phase they are currently vaccinating in, as well as other information. The survey is also an option for the provider to request additional doses as part of the allocation process. If you have not received the weekly survey, please email DPH-Vaccine-Management@massmail.state.ma.us.
Administrative Considerations
What requirements must the local board of health meet in order to offer a COVID-19 vaccination program for first responders?
All organizations or providers receiving COVID-19 vaccine must execute the Massachusetts COVID-19 Vaccine Program (MCVP) Agreement. Among other things, this Agreement obligates providers to administer COVID-19 vaccine in accordance with the terms of the United States Food and Drug Administration (FDA) Emergency Use Authorization (EUA) applicable to the vaccine that will be administered. The MCVP Agreement is emailed as a link to contacts associated with the Massachusetts Immunization Information System (MIIS) and State Vaccine Program.
Sites enrolling in the MCVP must already be registered with the MIIS. Pursuant to G.L. c. 111 s. 24M and 105 CMR 222, licensed healthcare providers who administer immunizations are required to report certain information to the Department’s MIIS. COVID-19 vaccine providers must meet this reporting requirement by registering with the MIIS, which will include executing the MIIS Site and User Agreements.
You must have the capacity to fully organize and staff the clinics to meet the vaccination needs of at least 200 first responders to qualify. This includes local capacity to fully organize and staff the clinics, to safely store vaccine, and to bill insurance for administration-related costs if other local financial resources are not available. At this time, no funding for administration related costs is available through the federal government or from the state. The Commonwealth will allocate COVID-19 vaccine and selected ancillary supplies, including syringes and needles, to approved local health departments, subject to available supply, but each location must be able to provide its own refrigeration/freezer capacity, PPE, clinical and non-clinical staffing, and any other resources needed to support clinic operations.
You must have the ability to schedule vaccination appointments and ensure that individuals will receive their second dose of vaccine within the prescribed time frame. If you have a system in place to schedule appointments, you may continue to use that, or you may use PrepMod, a web-based system offered through DPH.
What is PrepMod? (Updated 1/6/21)
PrepMod is an online, paperless system that you can use at your first responder vaccination clinic to schedule, screen, bill, and report to the MIIS. The system provides companion technologies that automate registration, planning, implementation, evaluation, recording, and reporting for mass vaccination and preparedness efforts. Use of PrepMod is not required, but there is a federal requirement that all COVID-19 vaccine data be reported to the MIIS within 72 hours, and PrepMod will facilitate that process. If you enter all necessary information into PrepMod, it will be sent automatically to the MIIS so there is no need to enter the information again to satisfy required MIIS reporting. If you have questions about PrepMod, please contact Prepmod.help@mass.gov.
Is there a cost to providers to receive and administer the vaccine?
There is no cost for the vaccine or ancillary kits. The U.S. Centers for Medicare and Medicaid Services (CMS) has approved reimbursement for the administration of the vaccine. While vaccine providers may not bill for the COVID-19 vaccine itself, many vaccine providers in the state have contracted with outside entities, such as Commonwealth Medicine, to assist with insurance billing for the costs of administering the vaccine. The Department is not a party to these agreements, but if your site has such an agreement you may wish to familiarize yourself with its terms.
Is there a cost to vaccine recipients?
Providers must administer COVID-19 Vaccine regardless of the vaccine recipient’s ability to pay administration fees or the recipient’s insurance coverage status. Providers may seek appropriate reimbursement from a program or plan that covers COVID-19 Vaccine administration fees for the vaccine recipient. Providers may not seek co-payment, reimbursement or any form of cost sharing, including through balance billing, from the vaccine recipient.
Is written consent needed for COVID-19 vaccination?
Informed consent is a vital process prior to the administration of a vaccine. DPH does not require a written informed consent form from vaccine recipients. However, vaccine providers should consult with their legal counsel regarding an appropriate informed consent process and what documentation may be recommended or required by their particular organization.
In order to make a vaccine clinic appointment in PrepMod, the individual making the appointment will be prompted to confirm the following:
The information I provided is correct.
I have been provided the COVID-19 EUA Fact Sheet for Recipients and Caregivers which has information about the risks and benefits of the vaccine. I will be able to ask questions at the time I receive my immunization.
I have the legal authority to and give consent for me and any other person(s) I registered to be vaccinated with the vaccine(s) above.
I give permission for my insurance company to be billed for the costs of administering the vaccine(s). The government is paying for the vaccine itself and I will not be billed for that portion of the cost of my immunization.
I understand that, as required by state law, all immunizations will be reported to the Department of Public Health Massachusetts Immunization Information System (MIIS). I can access the MIIS factsheet for Parents and Patients, at www.mass.gov/dph/miis, for information on the MIIS and what to do if I object to my or my family's data being shared with other providers in the MIIS.
What information do we need to provide about the US FDA Emergency Use Authorization (EUA) of the Moderna and Pfizer-BioNTech COVID-19 Vaccines?
The Department plans to make Moderna’s COVID-19 vaccine available to sites holding first responder vaccination clinics. Appropriate storage of and maintenance of cold chain for the vaccine will be necessary, and the Fact Sheet for Recipients and Care Givers must be made available to each individual receiving the vaccine.
The Moderna COVID-19 Vaccine Letter of Authorization (Letter) which describes the terms of the EUA, Fact Sheet for Healthcare Providers Administering Vaccine (provider fact sheet), and Fact Sheet for Recipients and Care Givers (recipient fact sheet) in addition to other related documents and translations of the fact sheet are available here: Moderna COVID-19 Vaccine | FDA. It is important to review the documents from the linked FDA site so that you have access to any updates or amendments.
Facilities, organizations, and healthcare providers holding vaccination clinics, as vaccination providers, should carefully review the Letter of Authorization and the provider and recipient fact sheets for the particular vaccine they will be administering. The Letter places obligations on vaccination providers including administering the vaccine in accordance with the EUA, making the recipient fact sheets available to each individual receiving the vaccine, and reporting certain information to the Vaccine Adverse Event Reporting System (VAERS).
In the event that it becomes necessary to provide the Pfizer-BioNTech COVID-19 vaccine for first responder clinics, the corresponding Letter and fact sheets in addition to other related documents and translations of the fact sheet should be carefully reviewed and are available here: Pfizer-BioNTech COVID-19 Vaccine | FDA. This Letter also places obligations on vaccine providers.
Site Considerations
What capacity does our site need to safely carry out COVID-19 vaccination best practices?
Screen patients for COVID-19 symptoms before and during the visit. Screening questions can be found in PrepMod, or sites can find sample forms on the CDC website.
Maintain physical distance (at least 6 feet apart, where possible).
Limit and monitor facility points of entry and install barriers to limit physical contact with patients at triage.
Observe respiratory hygiene (surgical facemasks for staff and face coverings for patients over 2 years of age, if tolerated) and cough etiquette.
Observe hand hygiene (including providing at least 60% alcohol hand sanitizer for patients).
Monitor individuals for possible adverse reactions. CDC recommends that persons without contraindications to vaccination who receive an mRNA COVID-19 vaccine be observed after vaccination for the following time periods:
30 minutes: Persons with a history of an immediate allergic reaction of any severity to a vaccine or injectable therapy and persons with a history of anaphylaxis due to any cause.
15 minutes: All other persons.
Perform enhanced surface decontamination. Detailed guidance for cleaning surfaces can found at this CDC site: https://www.cdc.gov/coronavirus/2019-ncov/community/disinfecting-building-facility.html
Refer to CDC guidance to prevent the spread of COVID-19 in health care settings, including outpatient and ambulatory care settings.
Ensure there is an adequate location to safely store the vaccine. Detailed information on safe storage and handling guidelines can be found here.
What type of refrigeration will we need?
LBOHs will be provided Moderna vaccine which can be stored at -15 to -25C for 6 months and at 2-8C for 30 days. DPH strongly encourages sites to store their Moderna vaccine frozen. Pharmaceutical and purpose-built refrigerators are a vaccine storage and handling best practice but are not required for the storage of COVID-19 vaccine. Standalone freezers are strong recommended, as the freezer portion of a household combination unit does not reliably maintain temperatures. If that is not possible for your site, please contact the Vaccine Unit at DPH-Vaccine-Management@massmail.state.ma.us for further guidance to ensure maintenance of appropriate temperatures. All storage units must be monitored continuously. The best practice for monitoring temperatures is to use a digital data logger available from DPH.
Operational Considerations
How should we set up our clinic?
Your current Emergency Dispensing Site (EDS) plan should serve as the foundation for standing up your first responder clinics. Review your plan to determine whether adjustments are necessary to support clinic operations and to provide for safe clinic set-up and flow. You can find the MDPH resource Emergency Dispensing Site (EDS): A Guide for Local Health On Planning for Medical Countermeasure (MCM) Dispensing Operations as well as other resources here.
If you are planning to set up a drive through clinic, please follow CDC guidance: Considerations for Planning Curbside/Drive-Through Vaccination Clinics | CDC. Please also note that the Interim Clinical Considerations for Use of mRNA COVID-19 Vaccine | CDC also state that for people with a history of any immediate allergic reaction to any other vaccine or injectable therapy, there should be an ability of the person to be vaccinated in a setting where appropriate medical care is immediately available for anaphylaxis.
How do sites that are holding first responder COVID-19 vaccination clinics order vaccine?
In Phase 1 of the Commonwealth’s COVID-19 Vaccine Plan, vaccine allocations will be determined by the MCVP; sites will not place orders. DPH will allocate vaccine to the site based on the availability of vaccine allocated by the federal government to Massachusetts, the information that the site provided in the MCVP Agreement, and information about the number of appointments scheduled. Once sites are able to place orders for vaccine directly, ordering will be done in the MIIS.
How do sites ask for more COVID-19 vaccine? (Updated 1/13/21)
On a weekly basis, all provider sites will receive a link to an MCVP survey. Completing this survey will ensure that DPH understands providers’ vaccine needs, the phase they are currently vaccinating in, as well as other information. Additionally, there is an option for the provider to also request additional doses as part of this survey process. If you have not received the weekly survey please email DPH-Vaccine-Management@massmail.state.ma.us.
How will sites that are holding first responder COVID-19 vaccination clinics receive the vaccine?
The Moderna COVID-19 vaccine will be delivered by UPS or FedEx to the sites you have identified to the MCVP. If working in a multi-community group, you may choose to have vaccine delivered to a single community and re-distributed to other community sites that meet all requirements for receipt of COVID-19 vaccine. Any community that will be receiving the vaccine either directly from UPS or FedEx or re-distributed through another community must be registered in the MIIS and have completed an MCVP Provider Agreement. In the MCVP Provider Agreement, ensure that you enter an accurate shipping address and shipping hours so that staff are on site to receive the vaccine shipment. We cannot guarantee that you will receive a phone call from the delivery driver when the shipment arrives.
To update your shipping address or hours, contact the Vaccine Unit at DPH-Vaccine-Management@massmail.state.ma.us Include your PIN and the contact email of who will update the Agreement. We will send out a link that will allow you to update the Agreement.
Can we take vaccine to EMS/police/fire stations rather than have them come to a clinic?
Yes, so long as you comply with all applicable requirements, including those for safe transport of vaccine. Consider the following general principles, which can also be found in the DPH COVID-19 Vaccine Training: The Moderna Supplement (PDF Slides).
Once a vial of vaccine has thawed, it may be stored refrigerated at 2° to 8° C for up to 30 days.
Once thawed, the vaccine cannot be re-frozen.
When thawed, the vaccine should be handled with care and protected from shocks, drops, vibration, etc.
Vaccine being transported at temperatures others than frozen (-15° to -25° C) should begin with the vaccine in the frozen state if at all possible.
If you must transport vaccine that has already been thawed, follow these general principles:
Punctured vials should not be transported.
Care must be taken to ensure vaccine does not re-freeze during transport.
Vaccine must be protected as much as possible from drops, shocks, and vibration whether in the carton, vial, case or cooler.
Vaccine should be transported in the carton whenever possible.
If transport must be conducted at the vial level, place the vial with dunnage (padding material like bubble wrap or similar padding) to minimize movement during transport.
The vaccine should always be transported in insulated containers qualified to maintain 2° to 8° C for the duration of transport.
The transport containers must be secured when being transported to prevent unnecessary movement.
After completion of transport, vaccine should immediately be placed into a vaccine storage unit at 2° to 8° C.
Vaccine should only be transported one time and should not be transported back again to the point of origin or to a new location.
Allowable timelines for transport of thawed vaccine are shown below. Total transport time should not exceed 12 hours in total.
Transport while walking or using hand cart: not to exceed 1 hour
Vehicle transport: not to exceed 12 hours
Airplane transport (rotary wing aircraft not permitted): not to exceed 3 hours
Will we need a standing order for the first responder clinics?
You will need to obtain a standing order for your program from a medical professional, such as a physician.
State law, M.G.L. c. 94C, section 8 (7), requires a licensed provider with prescribing authority to issue an order for administration of a vaccine such as the COVID-19 vaccine.
Authorized ordering providers include, a: physician, chiropractor, surgeon, podiatrist, osteopath, nurse practitioner, dentist, or physician’s assistant. See MGL Ch. 94C; 105 CMR 700.00.
A standing order is an order issued by a licensed provider, which is not specific to one person, and enables assessment and vaccination of patients without the need for clinician examination or direct order from the attending provider at the time of the interaction.
The standing order should be specific about which clinics and what dates or periods of time are covered, e.g., “COVID-19 vaccination clinics for first responders operated by (name of organization, LBOH, coalition, etc.) from January 4, 2021 through February 28, 2021.”
Any individual who meets the criteria included in a standing order may receive the vaccine consistent with the terms of the order.
A model standing order developed by CDC for the Moderna COVID-19 vaccine can be found here.
Other Emergency Treatment Standing order templates are available from the Immunization Action Coalition:
Medical Management of Vaccine Reactions of Adults in a Community Setting
Medical Management of Vaccine Reactions in Children and Teens in a Community Setting
How do we notify and schedule first responders for our clinic?
Appointments are strongly encouraged to ensure that there is no wasted vaccine and to help manage queuing and social distancing. If a site chooses to allow walk-ins, there is no guarantee that they will be able to be vaccinated.
If you have a system you currently use to schedule appointments, you can continue to use that. You must be able to generate a 2nd dose reminder for vaccine recipients through your electronic scheduling system or by written reminder. You will also need to manually enter the mandated data into the MIIS within the federally required 72 hours after administering vaccine. As an alternative, DPH will be offering PrepMod, which can be used to schedule, screen, bill, and report to the MIIS. PrepMod will be available through a link on the COVID-19 webpage, and first responders will be able to register for available clinics. If you use PrepMod, your clinic information and scheduling availability will be listed on the website. More information will be shared about PrepMod soon.
Do we need to have 200 confirmed appointments to receive vaccine?
You must have identified at least 200 first responders eligible for vaccination and have the capacity to vaccinate at least 200 first responders, but you do not have to have 200 confirmed appointments to receive vaccine.
What should a site do with extra vaccine (e.g. if we get more doses than we have first responders who are willing to be vaccinated)? (Updated 1/6/21)
Sites can continue to vaccinate anyone in a currently eligible priority group in accordance with the Commonwealth’s COVID-19 Vaccine Plan, so COVID-facing health care workers in the community (including vaccinators and COVID testers) can also be vaccinated with excess vaccine because both COVID-facing health care workers and first responders will be in currently eligible priority groups. Vaccine can also be transferred to small primary care practices, that meet all requirements for receipt of COVID-19 vaccine, for their eligible staff. Such a transfer must be done in consultation with the Vaccine Unit. Visit Massachusetts COVID-19 Vaccine Program (MCVP) – Guidance for Healthcare Providers and Organizations | Mass.gov for more guidance on redistribution.
Where will people receive their second dose?
The expectation is that the people will receive their second dose from the same clinic or other clinics in the same area. Alternatively, people can get their second dose at a mass vaccination clinic site or primary care provider. It is important for people to receive the second dose to ensure full efficacy. For COVID-19 vaccines requiring 2 doses, the 2nd dose must be the same vaccine product as the first dose.
How will people know which vaccine product they receive and when they need the second dose?
The vaccine ancillary supply kits will come with vaccine record cards that can be given to the recipients indicating what vaccine they received and that they need a second dose. Vaccine record cards may be reproduced, if necessary. In addition, there are electronic reminder/recall systems in the MIIS that providers could use in addition to their own EHR systems to send reminders to recipients about their second dose. More information about the v-safe app, which also includes a reminder recall, can be found at V-safe After Vaccination Health Checker | CDC. Providers should schedule the 2nd dose at the time the 1st dose is administered.
What vaccine and ancillary supplies will sites that are holding first responder COVID-19 vaccination clinics receive?
The first responder clinics will receive the Moderna COVID-19 vaccine. Hospitals that are working with local health to vaccinate their first responders will receive Pfizer. The Standard COVID-19 Vaccine Adult Ancillary Kit supports administration of 100 doses and includes needles, syringes, alcohol pads, vaccination record cards, needle guide, face shields, and face masks. Additional details about these supplies can be found at Massachusetts COVID-19 Vaccine Program (MCVP) – Guidance for Healthcare Providers and Organizations | Mass.gov.
Are we responsible for printing and making available information on v-safe? (Updated 1/12/21)
Yes. V-safe is a new voluntary, smartphone-based tool that uses text messaging and web surveys to provide personalized health check-ins for COVID-19 vaccine recipients. V-safe allows people to report any side effects after COVID-19 vaccination to CDC in almost real time. It also gives them a convenient reminder to get their second COVID-19 vaccine dose if they need one. Provide the v-safe Information Sheet or QR code to every vaccine recipient and encourage them to enroll and complete the surveys when prompted to do so. For more information, or to register for v-safe, visit: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html
Staffing Considerations
Who can administer vaccine? (Updated 1/13/21)
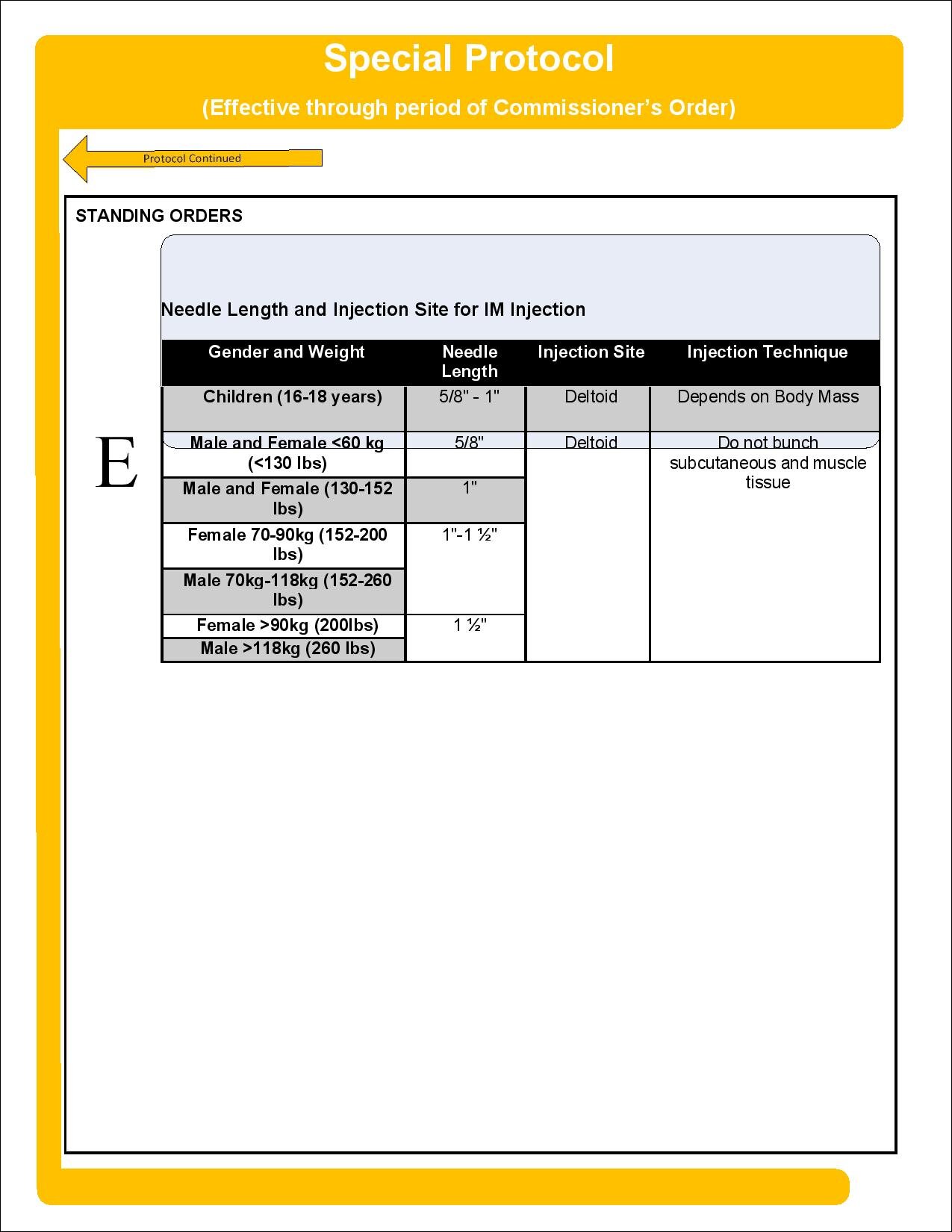
This COVID-19 Vaccinators chart lists the categories of health professionals who can possess and administer COVID-19 vaccines. EMTs and paramedics supporting vaccine clinics must be employed by a licensed ambulance service and be trained and operating with the approval of their Affiliate Hospital Medical Director. Please review the Office of Emergency Medical Services advisories and protocols.
What qualifications do individuals need to operate the program and administer the vaccine?
All individuals who receive vaccine deliveries, handle, or administer vaccines must be trained in vaccine related practices and procedures. They should be able to ensure the safety and efficacy of vaccines through proper:
Benefit and risk communication
Vaccine storage/handling and administration
Timing and spacing of vaccine doses
Screening for contraindications and precautions
Management of adverse reactions
Being able to access and use emergency equipment
Current CPR certification
Reporting to VAERS (and any additional COVID specific databases)
Documentation
If you will be using volunteer vaccinators for your clinics, make sure that everyone is up-to-date with their vaccinating skills. You can use the checklist below and, if needed, have the volunteers watch the training video.
Do sites that are holding first responder COVID-19 vaccination clinics always have to have one Vaccine Coordinator and one Backup Vaccine Coordinator on site for the duration of a clinic?
Yes, at least one of these individuals should be on site at all times.
Is there a training for vaccinators?
The primary and back-up vaccine coordinators at each site and providers administering COVID-19 vaccine are encouraged to complete the Introduction to COVID-19 Vaccine Storage & Handling and Administration Training, which has a focus on the Pfizer-BioNTech COVID-19 vaccine. Recording and slides from the training are below:
COVID-19 Storage and Handling Training PDF Slides | (Accessible)
COVID-19 Vaccine Administration Training PDF Slides | (Accessible)
Vaccine coordinators and providers are also strongly encouraged to complete the COVID-19 Vaccine Training: The Moderna Supplement. This training is designed as supplemental training to the COVID-19 Vaccine Storage & Handling and Administration training listed above. It includes updates and is designed for health care providers, vaccine coordinators, and all health care personnel who handle and/or administer vaccines. Recording and slides from the training are below:
What personal protective equipment (PPE) is needed?
Each site must provide its own PPE for clinic sites and ensure an adequate supply including:
Surgical Masks
Required: All health care providers (N95 masks not recommended)
Eye protection
Required: Areas of moderate/substantial community transmission or if ultra-cold/dry ice is being handled
Optional: Areas of minimal/no community transmission
Gloves
Required: Latex or similar gloves needed to administer intramuscular or subcutaneous vaccine
Required: If ultra-cold or dry ice are being handled, special insulating gloves are needed
Clinical Considerations
For both the Pfizer and Moderna vaccines, there may be enough extra in each vial for more than the standard 5 or 10 doses. Is it okay to administer additional doses from a vial? (New 1/7/21)
Yes. After preparation, vials of Pfizer-BioNTech COVID-19 Vaccine contain up to six doses of 0.3 mL. Low dead-volume syringes and/or needles can be used to extract up to six doses from a single vial. If standard syringes and needles are used, there may not be sufficient volume to extract a sixth dose from a single vial. Irrespective of the type of syringe and needle:
Each dose must contain 0.3 mL of vaccine.
If the amount of vaccine remaining in the vial cannot provide a full dose of 0.3 mL, discard the vial and any excess volume.
Do not pool excess vaccine from multiple vials.
As a reminder, use only the prescribed 1.8 ml of diluent when reconstituting a vial.
Vaccinators may also find that they can withdraw more than 10 doses of the Moderna COVID-19 vaccine from a single 10-dose vial.
Extra vaccine fluid from more than one vial CANNOT be combined to produce extra doses.
Use any extra vaccine that can easily be drawn into a syringe from one vial to meet the 0.5 ml dose requirement.
Enter all vaccine doses administered into the MIIS.
What should sites that are holding first responder COVID-19 vaccination clinics do with unused doses in the Moderna COVID-19 vaccine multi-dose vial?
Once a vial of Moderna COVID-19 vaccine has been entered, it must be used within 6 hours. Be sure to note the time the vial was first entered on the vial. Any vaccine remaining in the vial after 6 hours must be discarded.
Careful planning is important to ensure that COVID-19 vaccine is not wasted. It is essential that you have 10 people confirmed for vaccination within 6 hours before entering a new vial for the first. In the rare instance where you have COVID-19 vaccine that will expire and you have no one in the current priority groups to be vaccinated, you can use your clinical judgement to administer the vaccine to a person in another priority group who is closest to the current priority group being targeted for vaccination to avoid vaccine waste. It is important that you also ensure that this individual is now included in your reminder recall systems for the 2nd dose.
What are the potential side effects of the vaccine?
Systemic signs and symptoms, such as fever, fatigue, headache, chills, myalgia, and arthralgia, can occur following COVID-19 vaccination. Preliminary data from mRNA COVID-19 vaccine trials indicate that most systemic post-vaccination signs and symptoms are mild to moderate in severity, occur within the first three days of vaccination (the day of vaccination and following two days, with most occurring the day after vaccination), resolve within 1-2 days of onset, and are more frequent and severe following the second dose and among younger persons compared to those who are older (>55 years). Cough, shortness of breath, rhinorrhea, sore throat, or loss of taste or smell are not consistent with post-vaccination symptoms, and instead may be symptoms of SARS-CoV-2 or another infection.
Should someone who is COVID-positive receive the vaccine?
Vaccination of persons with known current SARS-CoV-2 infection should be deferred until the person has recovered from the acute illness (if the person had symptoms) and criteria have been met for them to discontinue isolation. While there is otherwise no recommended minimum interval between infection and vaccination, current evidence suggests that reinfection is uncommon in the 90 days after initial infection. Thus, persons with documented acute SARS-CoV-2 infection in the preceding 90 days may delay vaccination until near the end of this period, if desired.
Should people who have had COVID-19 be vaccinated?
Yes, people who have previously had COVID-19 should be vaccinated. Though it is uncommon to be re-infected in the 90 days after initial infection, people may choose to delay vaccination until the end of this period.
Can the vaccine be given to people who are pregnant?
There are no data on the safety of COVID-19 vaccines in people who are pregnant. Animal developmental and reproductive toxicity (DART) studies are ongoing. Studies in humans are also ongoing and more are planned.
If a person is part of a group (e.g., healthcare personnel) who is recommended to receive a COVID-19 vaccine and is pregnant, that person may choose to be vaccinated and may wish to discuss with their healthcare provider.
mRNA vaccines are not live vaccines. They are degraded quickly by normal cellular processes and don’t enter the nucleus of the cell.
COVID-19 infection during pregnancy can result in an increased risk of severe illness (ICU admission, mechanical ventilation and death) and might result in an increased risk of adverse pregnancy outcomes, such as preterm birth.
Consider the following when discussing COVID-19 vaccination with people who are pregnant:
Level of COVID-19 community transmission, (risk of acquisition)
Personal risk of contracting COVID-19, (by occupation or other activities)
The risks of COVID-19 to the person who is pregnant and potential risks to the fetus
The efficacy of the vaccine
The known side effects of the vaccine
The lack of data about the vaccine during pregnancy
Pregnant people who experience fever following vaccination should be counseled to take acetaminophen as fever has been associated with adverse pregnancy outcomes.
Routine testing for pregnancy prior to receipt of a COVID-19 vaccine is not recommended.