MA DPH Updated Comprehensive PPE Guidance
The Massachusetts Department of Public Health is releasing updated Comprehensive Personal Protective Equipment (PPE) Guidance. This guidance further updates the PPE standards for health care personnel. As part of this updated guidance, healthcare providers may implement policies to require HCP to use employer-issued PPE, and to preclude staff from using their own PPE absent appropriate safety and quality controls which may include but are not limited to fit-testing.
Memorandum
TO: Health Care Facility Chief Executive Officers and Administrators
Occupational Health Program Leaders
Emergency Medical Service Directors
FROM: Elizabeth Daake Kelley, MPH, MBA, Director
Bureau of Health Care Safety and Quality
SUBJECT: Comprehensive Personal Protective Equipment (PPE) Guidance
DATE: January 21, 2022
The Massachusetts Department of Public Health (DPH) continues to work with state, federal and local partners on the outbreak of Coronavirus Disease 2019 (COVID-19), caused by the virus SARS-CoV-2, and we continue to appreciate the essential role you have in responding to this evolving situation.
DPH has developed this comprehensive guidance, based upon the Centers for Disease Control and Prevention (CDC) recommendations, to clarify the PPE that health care personnel (HCP) use in clinical care areas and in other non-clinical areas in health care facilities. HCP refers to all paid and unpaid persons serving in healthcare settings and emergency medical services (EMS) who have the potential for direct or indirect exposure to patients or infectious materials including body substances; contaminated medical supplies, devices, and equipment; contaminated environmental surfaces; or contaminated air.[1] Healthcare settings are not limited to health care facilities but may include community settings where home health workers or EMS personnel are providing care to patients. This guidance further updates the PPE standards that HCP must follow. Healthcare providers may implement policies to require HCP to use employer-issued PPE, and to preclude staff from using their own PPE absent appropriate safety and quality controls which may include but are not limited to fit-testing.
These changes may be implemented immediately.
Universal Use of Facemasks
DPH has adopted a universal facemask use policy for all HCP. All HCP should don a facemask upon entry to the healthcare facility premises or care area (which includes ambulances); HCP working in community settings should don a facemask upon entry to the home or immediate area where they will be providing assessment and care. Facemasks are defined as surgical or procedure masks worn to protect the mouth/nose against infectious materials and have been shown to be highly effective at preventing transmission of COVID-19 when both individuals are masked. This policy will have two presumed benefits. The first benefit is to prevent pre-symptomatic spread of COVID-19 from HCP to patients, visitors and colleagues by reducing the transmission of droplets. The second benefit is to protect HCP by reducing transmission from their surroundings, including from other staff, visitors and patients who may be in a pre-symptomatic stage.
Extended use of facemasks is the practice of wearing the same facemask for repeated encounters with several different patients without removing the facemask between patient encounters. Due to improvement in the health care supply chain of facemasks, DPH is modifying earlier guidance and supports face mask use as follows:
As PPE to protect their nose and mouth from exposure to splashes, sprays, splatter, and respiratory secretions (e.g., for patients on Droplet Precautions). When used for this purpose, facemasks should be removed and discarded after each patient encounter.
As source control to cover one’s mouth and nose to prevent spread of respiratory secretions when they are talking, sneezing, or coughing. When used for this purpose, facemasks may be used for multiple patient encounters under the following conditions:
· The facemask should be removed and discarded if soiled, damaged, or hard to breathe through.
· HCP must take care not to touch their facemask. If they touch or adjust their facemask they must immediately perform hand hygiene.
· HCP should leave the clinical care area if they need to remove the facemask. (i.e., outside of the patient room)
· Facemasks should not be stored or put down on a surface; when removed, facemasks should be discarded and HCP should don a new facemask.
· If HCP remove their facemask to eat, drink or during a break they should perform hand hygiene with soap and water or an alcohol-based hand rub before and after touching their mask.
Homemade and cloth facemasks are not considered PPE and are not appropriate for use in the healthcare setting or by HCP.
As part of universal source control, if tolerated, patients/residents should wear a facemask issued by their provider when they leave their room or when staff are providing care to them.
PPE for patients with suspected or confirmed COVID-19, or confirmed exposures to COVID-19
DPH recommends that a fit-tested N95 filtering facepiece respirator or alternative, eye protection, isolation gown and gloves be used when caring for patients with suspected or confirmed COVID-19 or confirmed exposure to COVID-19.
Respirators:
Proper use of respiratory protection by HCP requires a comprehensive program (including medical clearance, training, and fit testing) that complies with OSHA’s Respiratory Protection Standard.
Facilities should eliminate the practice of reuse of N95 respirators with the exception of conditions outlined in Other Considerations. N95 respirators should always be discarded after doffing, such as when leaving a patient room, during a break or when eating or drinking. Respirators contaminated with blood, respiratory or nasal secretions, or other bodily fluids must be discarded immediately.
If reusable N95 respirator alternatives such as elastomeric respirators are used each facility must ensure appropriate cleaning and disinfection between uses and filter exchange according to manufacturer’s instructions. When used as source control, reusable N95 respirator alternatives may be worn between patients seen sequentially without cleaning and disinfection. If worn when seeing patients on transmission-based precautions, extended use may be performed if not contaminated.
Eye Protection:
At this time, when the risk of community transmission of COVID-19 in Massachusetts continues to be increased, HCP should wear eye protection for all patient encounters.
Disposable eye protection should be removed and discarded when it is removed for any reason; it should not be reused. Reusable eye protection should be cleaned and disinfected when visibly soiled and after removal/doffing of eye protection. Eye protection may be used for multiple patient encounters under the following conditions:
Eye protection should be removed and reprocessed if it becomes visibly soiled or difficult to see through.
Eye protection should be discarded if it becomes damaged (e.g., face shield can no longer fasten securely to the provider, if visibility is obscured and reprocessing does not restore visibility).
If reusable goggles or face shields are used each facility must ensure appropriate cleaning and disinfection between uses according to manufacturer’s instructions.
After cleaning and disinfection, reusable eye protection should be stored in a designated location.
HCP should not touch their eye protection while being worn. If they touch or adjust their eye protection hand hygiene must be performed.
HCP should leave the clinical care area if they need to remove their eye protection using recommended protocols for removing, cleaning, and disinfecting, and reprocessing.
Isolation Gowns:
Nonsterile, disposable patient isolation gowns, which are used for routine patient care in healthcare settings, are appropriate for use by HCP when caring for patients with suspected or confirmed COVID-19 or confirmed exposure. HCP may also use reusable (i.e., washable) gowns made of polyester or polyester-cotton fabrics; they can be safely laundered according to routine procedures and reused. Reusable gowns should be replaced when thin or ripped, and per the manufacturer’s instructions. Gowns should be disposed of or laundered after each patient encounter.
Any gown that becomes visibly soiled during patient care should be disposed of or laundered, as appropriate.
Gloves:
HCP should perform hand hygiene prior to donning and after doffing gloves.
Other Considerations:
For performing aerosol generating procedures, such as nebulizer treatments or intubations, HCP should don a fit-tested N95 filtering facepiece respirator or acceptable alternate product except in the following circumstances when Standard Precautions may be used:
The patient has recovered from COVID-19 within the previous 90 days;
The patient is asymptomatic, and a COVID-19 test obtained within the past three days is negative.
Health care organizations and providers that are caring for high numbers of patients with suspected or confirmed COVID-19, or confirmed exposures to COVID-19 during high rates of community transmission may choose to adopt any of the following principles when caring for patients in the same cohort (i.e. all confirmed COVID-19 cases):
Utilize the same N95 respirator or other acceptable alternate product between multiple patient encounters provided that the N95 respirator or acceptable alternative is always discarded after doffing, during a break, when eating or drinking or when contaminated with blood, respiratory or nasal secretions, or other bodily fluids.
Reuse the same N95 respirator or acceptable alternate product over the course of an entire shift; N95 respirators or acceptable alternative must be discarded if non-intact or contaminated with blood, respiratory or nasal secretions, or other bodily fluids.
Utilize the same eye protection between multiple patient encounters provided that the eye protection is clean and disinfected after doffing, or when contaminated with blood, respiratory or nasal secretions, or other bodily fluids.
Resources:
Health care organizations and providers that require additional PPE in order to meet the use standards described in this guidance and are not able to obtain through their usual supply chain resources may request support from DPH as a bridge until health care organizations increase their ordering and receipt of gloves, eye protection, facemasks, gowns and N95 respirators. DPH will review requests and provide additional PPE on a monthly basis for the months of January, February and March as a bridge supply for health care organizations and providers that have an immediate and insufficient supply for HCP caring for individuals with suspected or confirmed COVID-19 or exposures to COVID-19. Every health care organization must immediately adjust their supply order to ensure that going forward they have sufficient supplies to meet this guidance. A health care organization or provider who has insufficient supply should fill out and download the PPE request form and submit it via email to Covid19.resource.request@mass.gov.
The form may be found on DPH’s website:
https://www.mass.gov/info-details/personal-protective-equipment-ppe-during-covid-19. Please visit
DPH’s website that provides up-to-date information and guidance documents on COVID-19 for healthcare providers and organizations in Massachusetts:
Updated Comprehensive PPE Guidance
Dear Colleagues,
The Massachusetts Department of Public Health (DPH) is sharing updated Comprehensive Personal Protective Equipment (PPE) guidance. DPH is updating this guidance to:
Create greater flexibility to allow extended use of eye protection to align with CDC PPE use guidance issued at the end of September. DPH has clarified that reusable eye protection does not need to be cleaned in-between each patient encounter but as directed by the manufacturer and any time it is removed.
Update aerosol-generating procedure precautions based upon continued high levels of community spread. Providers must don N95 respirators or alternatives to perform these procedures unless there is a COVID-19 test within the past three days or the patient has recovered from COVID-19 within the past three months.
Clarify that this guidance applies to EMS personnel and home health workers in community settings.
Thank you for your ongoing dedication to your staff and patients throughout the COVID-19 pandemic.
Thank you,
The Massachusetts Department of Public Health
Comprehensive Personal Protective Equipment (PPE) Guidance
Recently, the Centers for Disease Control and Prevention (CDC) updated its recommendations for personal protective equipment (PPE). Based upon updated recommendations shared by the CDC, the attached updated guidance document from the Massachusetts Department of Public Health (DPH) updates PPE recommendations for health care providers to reflect a greater availability of PPE and return to conventional uses of PPE, including N95 respirators. Healthcare providers should expect to return to conventional facemask and respirator use, meaning discarding as mask after each patient encounter, no later than July 1, 2021.
Two Updates from DPH/OEMS
New PPE Preservation Planning Toolkit Now Available
The HHS Office of the Assistant Secretary for Preparedness and Response (ASPR) has developed a new toolkit, including links to a guide and an Excel spreadsheet, to assist organizations with planning and implementation strategies for personal protective equipment (PPE) preservation. Developed by ASPR’s COVID-19 Healthcare Resilience Working Group, the toolkit can help users understand types of PPE preservation strategies and calculate how using those strategies can increase the duration of a specified PPE supply.
MDPH COVID-19 PPE Stockpile Survey
Massachusetts Department of Public Health (MDPH) in collaboration with Massachusetts General Hospital Center for Disaster Medicine (MGH) and the Massachusetts Institute of Technology Humanitarian Supply Chain Lab (MIT) have developed the MDPH COVID-10 PPE Stockpile Survey, which is intended to inform the development of a state-level cache of PPE designed to support the emergent/urgent needs of Massachusetts healthcare organizations. Robust participation by healthcare organizations within Massachusetts will enable the development of a plan that can best meet the diverse needs of our collective organizations. Therefore, we would like to ask that you please take a moment to complete the survey for your organization.
Below are the link to the survey as well as a QR code to the survey.
Survey link https://www.surveymonkey.com/r/G53GPWM
Questions or feedback can be directed to Roberta Roberta.Crawford@Mass.gov
UPDATED Comprehensive Personal Protective Equipment (PPE) Guidance
The Massachusetts Department of Public Health continues to work with state, federal and local partners on the outbreak of Coronavirus Disease 2019 (COVID-19), caused by the virus SARS-CoV-2, and we continue to appreciate the essential role you have in responding to this evolving situation.
The Department of Public Health has developed updated comprehensive PPE guidance (see attached), based upon changes made to the Centers for Disease Control and Prevention recommendations about eye protection.
Comprehensive Personal Protective Equipment (PPE) Guidance
The Department of Public Health has released updated Comprehensive Personal Protective Equipment (PPE) Guidance for healthcare facilities. This updated guidance is also posted on the DPH’s COVID-19 website: Personal Protective Equipment (PPE) During COVID-19: Guidance.
Deadline for Battelle Critical Care Decontamination System N95 Masks THIS SUNDAY
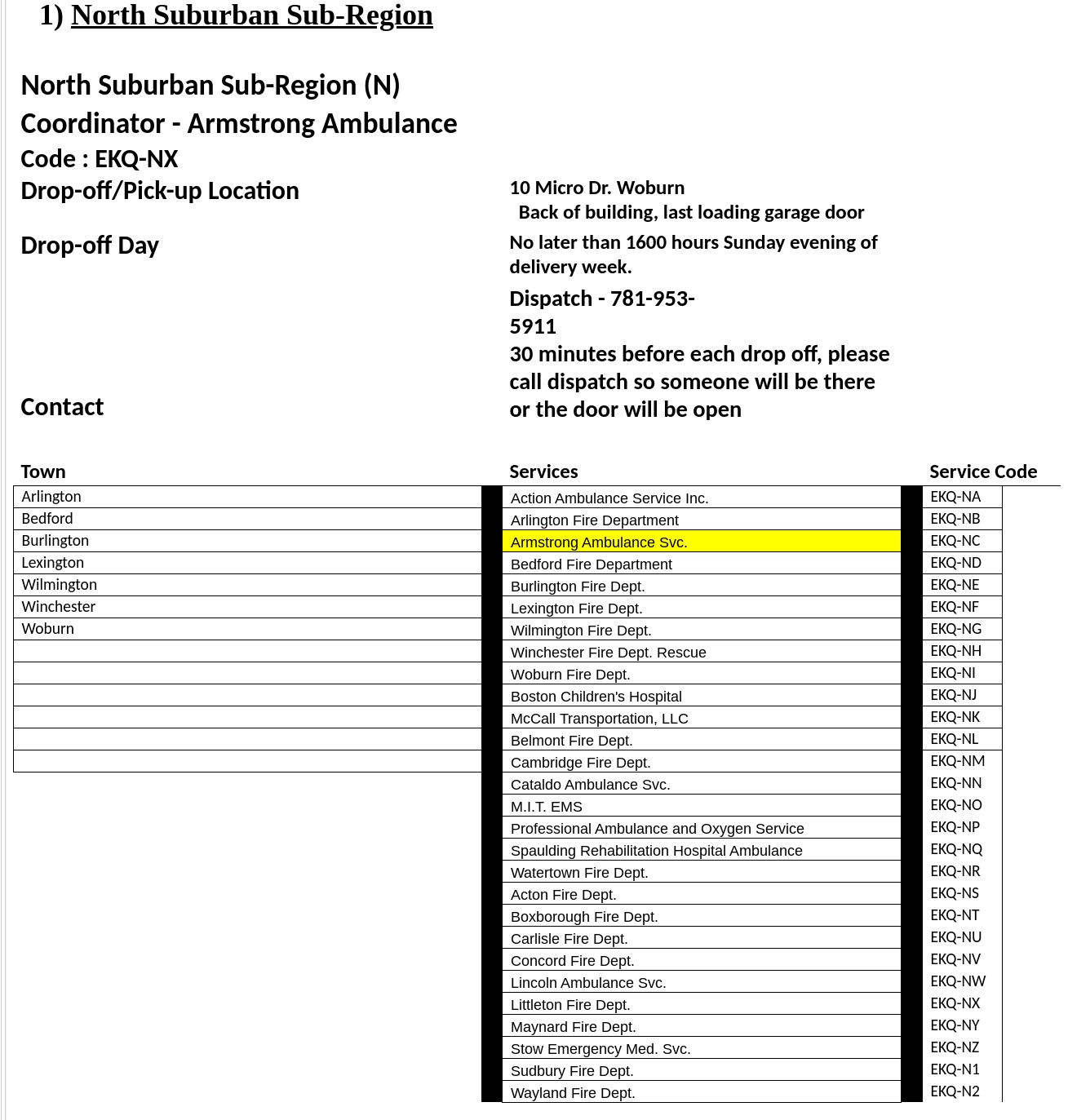
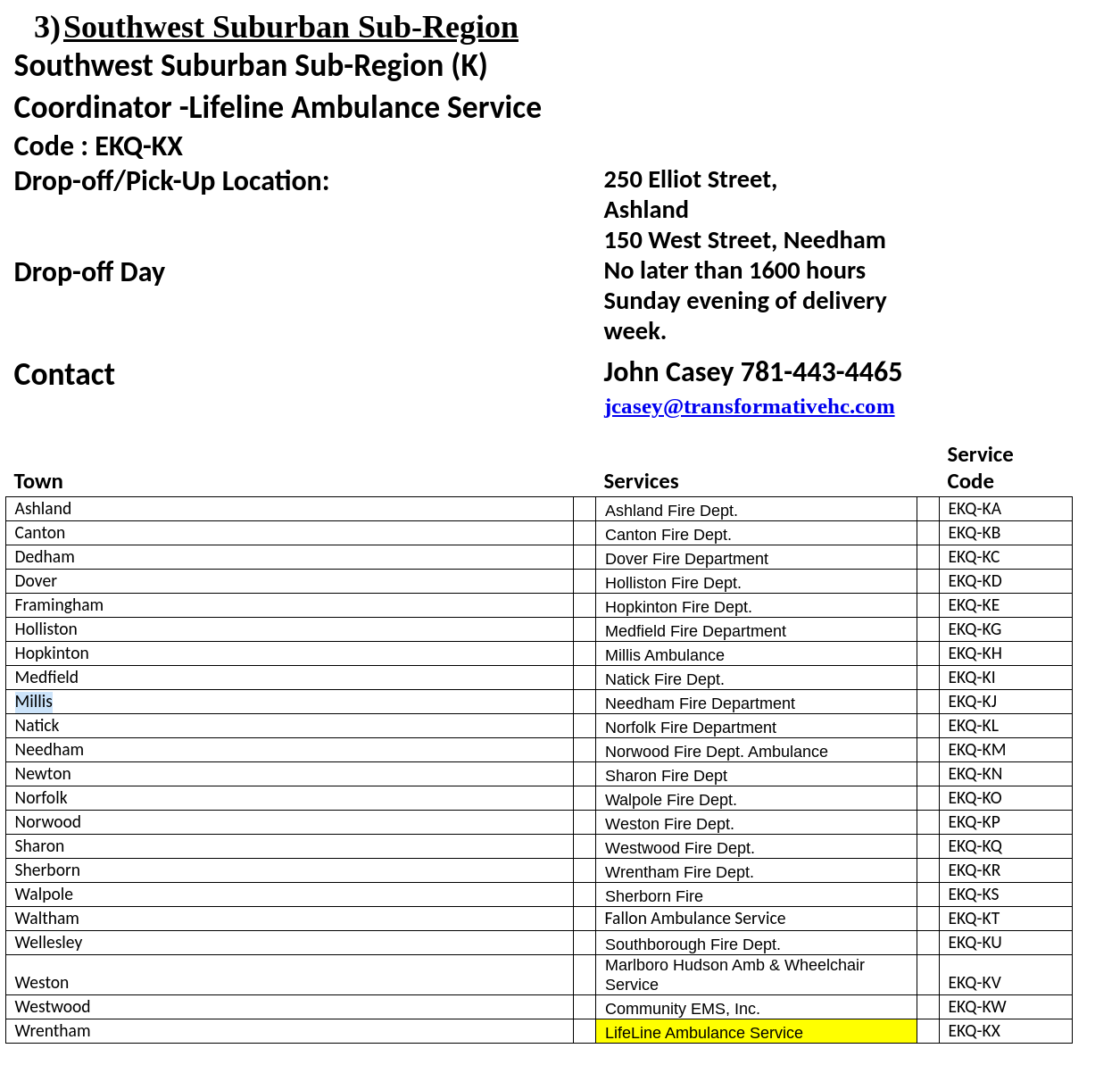
The collection period the deadline for this collection is Sunday May 3rd, no later than 1600 hours, with the exception of the South Suburban Sub-Region as indicated on their information below.. You will need to contact the sub-region designated point of contact prior to delivery to ensure that someone will be there to receive your delivery. Please see the following Region IV Sub-region designation form for points of contact, delivery locations, and service identification codes.
Should you have questions regarding any of the material, please contact Derrick Congdon, Executive Director, via email at dcongdon@mbemsc.org.
Instructions for Region IV EMS Services and Sub-region Coordinators: Preparation and Collection of Compatible N95 Respirators for Decontamination by the Battelle Memorial Institute Using the Battelle Decontamination System
The U.S. Food and Drug Administration has authorized an Emergency Use Authorization (EUA) for the emergency use of the Battelle CCDS Critical Care Decontamination System™ (hereafter referred to as the “Battelle Decontamination System”) operated by the Battelle Memorial Institute (“Battelle”), for use in decontaminating compatible N95 or N95-equivalent respirators (“compatible N95 respirators”), for reuse by healthcare personnel. Healthcare personnel should follow these instructions, as well as procedures at their healthcare facility, to prepare compatible N95 respirators for decontamination by Battelle using the Battelle Decontamination System.
• Due to incompatibility, the Battelle Decontamination System is not authorized for use with respirators containing cellulose-based materials
• All compatible N95 respirators provided to Battelle must be free of any visual soiling or contamination (e.g., blood, bodily fluids, makeup)
• If N95 respirators are soiled or damaged, they will be disposed of and not returned after decontamination
INSTRUCTIONS
EMS SERVICE AND EMTs
On-Site Collection/Marking
1. EMS services should create a collection station at the point of generation (i.e. garage/ambulance base)
2. Each station should have a bag provided by the service to collect compatible N95 respirators. NOTE: Bags are for compatible N95 respirators only. Do not throw other personal protective equipment (such as gloves), paper towels, or waste in the collection bag.
3. With a permanent marker, the EMS staff should label their own individual compatible N95 respirators with a three-digit site code and a 2-digit location identifier (as shown below). The unique three (3) digit site code corresponds to the Metropolitan Boston EMS Council (Region IV). The two (2) digit location identifier corresponds to a specific sub-region with in Region IV and the individual EMS service.
4. EMS staff should follow the instructions provided by Battelle in Instructions for Healthcare Personnel: Preparation of Compatible N95 Respirators for Decontamination by the Battelle Memorial Institute Using the Battelle Decontamination. (See Attached Instructions for Healthcare Personnel)
Region Code Sub-region/Service Code
EKQ - SA
Preparation for Shipment by EMS Service:
1. Bags containing the contaminated compatible N95 respirators to be decontaminated by Battelle (“primary collection bag” provided by the EMS service) should be closed.
2. Place the primary collection bag into another bag (“secondary collection bag” provided by the EMS service), which is then closed.
3. Decontaminate the secondary collection bag with alcohol or other suitable decontaminant.
4. Place the decontaminated bags into a rigid, closed box (i.e. cardboard), supplied by the EMS service, clearly labeled with a biohazard symbol, and tape the box securely shut.
5. Label the outside of the box with the 3-digit region code and 2-digit location identifier.
6. Complete a Region IV Chain of Custody Form for your EMS Service.
7. Deliver the completed box to the designated Region IV sub-region coordinator for transport to Battelle. All deliveries to the sub-region coordinators must be completed by 16:00 hours, the Sunday evening before transfer date to Battelle beginning April 26, 2020. This will be the first delivery to Battelle. Once we determine the number of PPE processed during this shipment, we will determine if collections will be weekly or bi-weekly moving forward.
SUB-REGION COORDINATOR
Sub-region Shipment of completed boxes is under the Metropolitan Boston EMS Council agreement with Battelle:
1. Gather all boxes at designated sub-region location (see attached spreadsheet for your exact Sub-region location); completed one chain of custody form (provided by Battelle) per shipment, noting the number of boxes.
2. Transfer boxes to designated Battelle location in Somerville, Massachusetts for decontamination.
3. Transfer decontaminated PPE from Battelle location in Somerville, Massachusetts back to sub-region for redistribution.
4. Notify Region when decontaminated PPE has been returned from Battelle, so the services in the sub-region can be notified to pick-up at the designated location.
Reuse Information:
Following decontamination, you will be provided decontaminated compatible N95 respirators that have been processed through a decontamination system for reuse by healthcare personnel in a healthcare setting during the COVID-19 pandemic. Before reuse, the healthcare facility should review the chain of custody form, which indicates successful decontamination, accompanying the returned respirators. The healthcare facility should also inspect each returned, decontaminated compatible N95 respirator for:
1. Numeric indication of the decontamination cycle number. NOTE: Compatible N95 respirators will be disposed of after 20 decontamination cycles.
2. Visible damage or soiling. NOTE: Compatible N95 Respirators should be discarded and not reused if visually damaged or soiled.
Any problems should be immediately reported to Battelle and the Metropolitan Boston EMS Council.
Battelle Contact: 1-800-201-2011 or solutions@battelle.org
Metropolitan Boston EMS Council Contact: 781- 505-4367 or dcongdon@mbemsc.org
Guidance for EMS on the Use of PPE in Long Term Care Facilities
The purpose of this communication is to share new guidance on the use of Personal Protective Equipment (PPE) by First Responders and EMS when entering a nursing home or rest home (long term care facility).
In alignment with the Centers for Medicare and Medicaid Services (CMS) and due to the widespread community transmission of COVID-19, the Department of Public Health (DPH) is advising all first responders, including but not limited to, fire personnel, law enforcement, and EMS personnel, to assume when responding to an event at a long term care facility that the individuals they are interacting with are infected with COVID-19. First responders and EMS personnel should don the appropriate PPE recommended for caring for individuals who are infected with COVID-19.
There is also a document with Nursing Home Frequently Asked Questions (FAQs) for First Responders & Municipalities.
OEMS Issues Comprehensive Personal Protective Equipment (PPE) Guidance
The purpose of this communication is to share comprehensive guidance, based upon the Centers for Disease Control and Prevention (CDC) recommendations, to clarify the Personal Protective Equipment (PPE) that health care personnel (HCP) use in the clinical care areas, particularly during this time when we are optimizing our supplies.
This guidance includes information about facemasks, eye protection, isolation gowns, gloves, respirators, and PPE for COVID-19 Patient Care.
The Massachusetts Department of Public Health (DPH) continues to work with state, federal and local partners on the outbreak of Coronavirus Disease 2019 (COVID-19), caused by the virus SARS-CoV-2, and we continue to appreciate the essential role you have in responding to this evolving situation.
UPDATED INFORMATION - REGION IV PPE CACHE BEING RELEASED
The Metropolitan Boston EMS Council, Inc (MBEMSC) in 2010 purchased a regional cache of PPE using funds from a private grant. The intent of the cache was to be able to provide Region 4 EMS services with access to PPE in the time of a severe supply chain demand issue for service providers unable to receive ordered supplies through their routine vendors during a pandemic or other incident. MBEMSC purchased enough PPE for 25,000 patient encounters. The supply of PPE includes gowns, surgical masks, and goggles. While the cache does have N95 masks in them they will not be released, initially due to expiration. The Council is actively working with the FDA, CDC, and NIOSH to expand the shelf-life of the N95s.
As of March 16, 2020 the Metropolitan Boston EMS Council has elected to release its PPE cache based on these guiding principles:
1. The cache is for MBEMSC EMS Services only. While many entities across the state are short on supplies, this cache was implemented for Region-4 EMS distribution only. As such, only the EMS services that fall within Region 4 are eligible to participate.
2. The cache must be replenished. When the supply chain is restored, replacement PPE will be ordered.
3. In order to replenish the inventory, participating services will need to purchase the cache items. Since the cache was developed with private funds (and not state/federal funds), services wishing to participate will be required to purchase the supplies they receive. Pricing is based on industry pricing. Items will be sold at market value incorporating estimated associated costs such as shipping/handling.
4. Expired items will not be distributed, initially. This only applies to N95s, which are all expired. MBEMSC is actively working with the FDA, CDC, and NIOSH to expand the shelf-life of the N95s that are in the cache. Likely, the only way these will be used is if all other methods of N95s and surgical masks are depleted nationally.
5. Distribution must be equitable. Distribution of the cache will be based on an EMS services 2019 call volume. Many (if not all) services are facing various degrees of shortages. To best gauge need & risk, current PPE status will not be factored into the distribution. Instead, current PPE status is being collected to provide state and federal resources with a more accurate summary of our regional needs.
6. Distribution will be performed once. To facilitate the rapid release of supplies, once the quantity of supplies allocated per service is calculated, all available supplies will be released. It is important to note that some services may not immediately need all items that they are allotted. In the event your service is allotted supplies you do not need, please notify us prior to payment/receipt of supplies so that redistribution can be evaluated.
7. Payment must be received before receipt of supplies. To ensure the future replacement of the cache, once your service’s allotment has been determined, you will receive an order form with the amount due. Prior to receiving your supplies, you will need to submit payment to MBEMSC in the form of check or money order.
In order to participate in the cache release, please submit this survey by 12:00 PM on Wednesday, 03/18/2020. https://www.surveymonkey.com/r/MBEMSC-PPE-03162020
We’re pleased to be able to offer this service. If you have any questions, don’t hesitate to call our Office.
COVID-19 Updates - CDC for EMS Personnel
Important updates from the Centers for Disease Control and Prevention (CDC) on interim guidance for EMS personnel who anticipate close contact with individuals with suspected or confirmed novel Coronavirus 2019 (COVID-19) in the course of their work:
The interim guidance has been updated based on currently available information about COVID-19 and the current situation in the United States, which includes reports of cases of community transmission, infections identified in healthcare personnel (HCP), and shortages of facemasks, N95 filtering facepiece respirators (FFRs) (commonly known as N95 respirators), and gowns.
The full guidance is available online at https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-for-ems.html. Please share this guidance broadly with EMS personnel working for your ambulance service.
Key points from the guidance updated on March 10, 2020 include the following:
Early reports suggest person-to-person transmission most commonly happens during close exposure to a person infected with COVID-19, primarily via respiratory droplets produced when the infected person coughs or sneezes. Airborne transmission from person-to-person over long distances is unlikely.Based on local and regional situational analysis of PPE supplies, facemasks are an acceptable alternative when the supply chain is limited.When shortages have been identified, available respirators should be prioritized for procedures that are likely to generate respiratory aerosols, which would pose the highest exposure risk to EMS and healthcare personnel.Eye protection, gown, and gloves continue to be recommended. If there are shortages of gowns, they should be prioritized for aerosol-generating procedures, care activities where splashes and sprays are anticipated and high-contact patient care activities that provide opportunities for transfer of pathogens to the hands and clothing of EMS and healthcare personnel. When the supply chain is restored, fit-tested EMS clinicians should return to use of respirators for patients with known or suspected COVID-19. Clean and disinfect the vehicle in accordance with standard operating procedures. All surfaces that may have come in contact with the patient or materials contaminated during patient care should be thoroughly cleaned and disinfected using an EPA-registered hospital grade disinfectant in accordance with the product label.The Environmental Protection Agency (EPA) has updated guidance about recommended EPA-registered disinfectants that meet the criteria for use against SARS-CoV-2, which is posted online at: https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2.